MICHIGAN — A few days after the U.S. Food and Drug Administration (FDA) authorized emergency use of the Pfizer COVID-19 vaccine, trucks carrying the vaccine left the company’s plant in Portage, Michigan on Sunday.
An estimated 2.9 million vaccines are expected to be delivered this week, with priority given to health care workers and aging and vulnerable Americans. Various health departments and hospital systems, including Henry Ford and Beaumont, are awaiting the doses, which will be housed in special freezers needed to store the vaccine.
UPS and FedEx will be delivering the vaccines, as COVID-19 case totals hit 16.2 million with 298,000 deaths.
High-risk populations in Michigan and the rest of the country will see the first doses, as is the case for the U.K.’s vaccine program which began last week. The vaccine will be rolled out in phases, with hospital workers and nursing home staff and residents included in the first phase.
In the U.K., the first round of vaccines saw a speed bump, with health officials noting adverse reactions in people with serious allergies to vaccines and other medicines, after at least two healthcare workers had allergies to the vaccine.
But FDA scientists “feel comfortable” that Americans should get the vaccine unless they have known allergies to other vaccines or the ingredients in the Pfizer vaccine. Those Americans are recommended to speak to their doctors about the COVID-19 vaccine.
“About 1.6 percent of the population has had a severe allergic reaction of some sort or another to a food or some environmental aspect,” said Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research. “We would really not like to have that many people not be able to receive the vaccine.”
Michigan priority plan
On Friday, Dr. Joneigh Khaldun, chief medical executive and chief deputy for health at the Michigan Department of Health and Human Services (MDHHS) and Robert Swanson, MDHHS’ Division of Immunizations director, gave insight into distribution of the vaccine, priority groups for vaccination and efficacy and safety information currently available for the vaccine.
The COVID-19 vaccines by Pfizer, Moderna and other have been developed faster than any vaccine in history, thanks to unprecedented, worldwide collaboration among scientists, medical doctors, health and government officials, and manufacturers. This collective effort has allowed researchers to shorten the typical vaccine timeline without sacrificing safety or quality.
The Pfizer vaccine is noted to have normal side effects associated with other vaccines, including redness and soreness at the inject site, fatigue, and mild fever.
The MDHHS’ goal is to immunize 70 percent of Michigan adults, or 5.4 million, by the end of 2021. It has also released its priority plan for the state’s immunization program:
Phase 1A includes paid and unpaid persons serving in health care settings who have direct or indirect exposure to patients or infectious materials and are unable to work from home, as well as residents of long-term care facilities.
Phase 1B includes some workers in essential and critical industries, including workers with unique skill sets such as non-hospital or non-public health laboratories and mortuary services.
Phase 1C includes people at high risk for severe COVID-19 illness due to underlying medical conditions and people 65 years and older.
Phase 2 is a mass vaccination campaign for all adults.
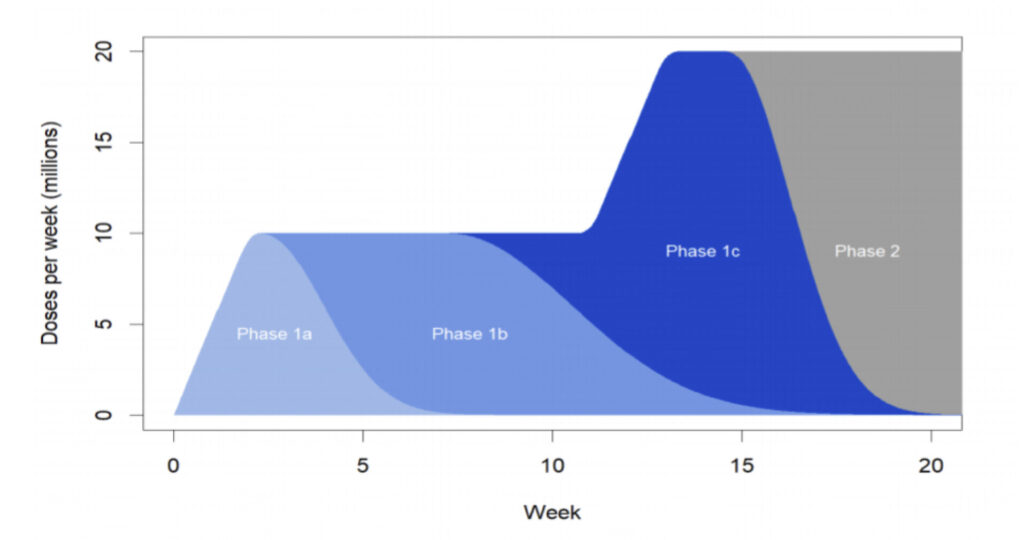
Source: MDHHS
Phase 1B will include K-12 school and child-care staff with direct contact with children and essential workers in many industries and service areas. Phase 1C will include vulnerable populations such as individuals age 65 years and older and individuals over age 18 with COPD, hypertension, chronic kidney disease, heart disease, diabetes, obesity or other conditions that puts them at high risk of negative COVID-19 outcome.
There will likely be overlap between each phase’s roll out. Hospitals, pharmacies, local health departments, EMS, the National Guard and and outpatient clinics will administer the vaccine. Communities hardest hit with the virus, like Wayne County, will be targeted for timing and distribution.
Moderna is awaiting approval of its vaccine by the FDA, which is expected to occur in the coming weeks, and will add to the vaccination numbers by millions.






Leave a Reply