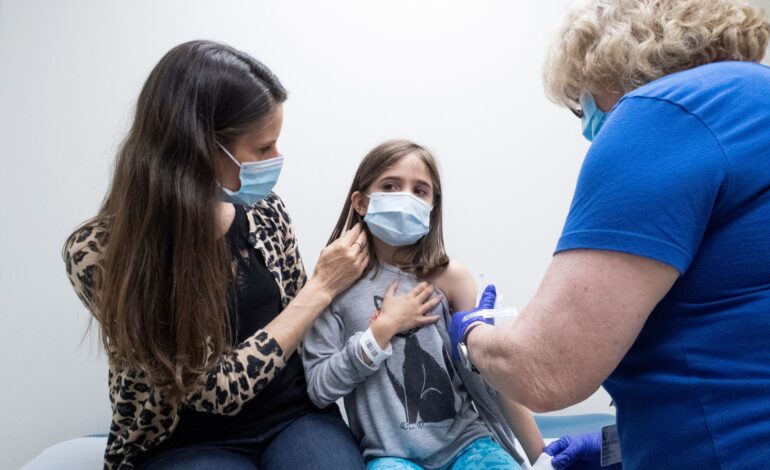
U.S. regulators authorized Pfizer and BioNTech’s COVID-19 vaccine for use in children as young as 12 and said they could begin receiving shots as soon as Thursday, widening the country’s inoculation program as vaccination rates have slowed significantly.
This is the first COVID-19 vaccine to be authorized in the U.S. for ages 12 to 15. Vaccinating younger ages is considered an important step for getting children back into schools safely. President Biden has asked states to make the vaccine available to younger adolescents immediately.
Biden issued a statement hailing the authorization as “a promising development in our fight against the virus.”
“If you are a parent who wants to protect your child, or a teenager who is interested in getting vaccinated, today’s decision is a step closer to that goal,” he said.
The vaccine has been available under an emergency use authorization to people as young as 16 in the U.S. The vaccine makers said they had started seeking full approval for the immunization in people 16 and older last week.
Peter Marks, director of the U.S. Food and Drug Administration’s (FDA) Center for Biologics Evaluation and Research, told reporters that states will likely be able to begin vaccinating 12- to 15-year-olds after an advisory committee of the U.S. Centers for Disease Control and Prevention (CDC) considers the expansion on Wednesday.
Most children with COVID-19 only develop mild symptoms or no symptoms at all. However, children are not without risk of becoming seriously ill, and they can still spread the virus. There have been outbreaks traced to sporting events and other activities for children in this age range.
“A godsend”
Dr. William Gruber, a top vaccine scientist at Pfizer, said the authorization of the vaccine for young teens would help the U.S. expand its immune population and protect an age group that has not been completely spared from severe disease.
“I hear from pediatricians and people out in the community, what a godsend this is going to be for the adolescent population who have been restricted in terms of sports activities, drama club and the other sorts of things that naturally we want them to engage in,” Gruber said.
Vaccines are crucial to ending the pandemic. But many health officials are concerned that vaccine hesitancy in some adults will be even more pronounced when it comes to their children.
Parents may question the risks versus benefits, given the unknowns about the vaccines’ long-term impact on children’s development and the low numbers of young kids who have been hit hard by COVID-19.
The companies said in March that they found the vaccine produced robust antibody responses and was safe and effective in 12- to 15-year olds in a clinical trial.
In the trial of 2,260 adolescents aged 12 to 15, there were 18 cases of COVID-19 in the group that got a placebo and none among those who received the vaccine, resulting in 100 percent efficacy in preventing the illness, the companies said at the time.
Around 46 percent of people in the U.S. have received at least one shot of a COVID-19 vaccine, according to data from the CDC.
But the pace of U.S. vaccinations has slowed significantly since peaking at a seven-day average of more than 3.3 million doses a day in mid-April. That average had fallen by more than a third to around 2.1 million shots a day as of May 4, according to CDC data.
Pfizer’s vaccine is the only one authorized for 16- and 17- year-olds in the U.S. Nearly 2 million people in that age group have received at least one shot, according to CDC data. Many states only opened to non-high risk individuals in that age group in early April.
Widely vaccinating 12- to 18-year olds could allow U.S. schools and summer camps to relax masking and social distancing measures suggested by the CDC.
Pfizer has said it expects to have safety and efficacy data on the vaccine for children ages 2 to 11 in September, when it plans to ask for that age group to be included in the emergency authorization.
– Reuters report, edited for style





Leave a Reply