Top U.S. health officials said on Wednesday that the protection offered by current available vaccines against COVID-19 wanes over time, as federal agencies have begun preparing to offer booster shots to all eligible Americans beginning the week of Sept. 20.
The booster shots will have to be administered starting eight months after a second dose of Pfizer or Moderna’s vaccines for most people, officials said. At that time, individuals who were fully vaccinated earliest in the vaccination rollout, including many health care providers, nursing home residents and other seniors, will likely be eligible for a booster.
Wednesday’s joint statement posted on the U.S. Food and Drug Administration (FDA) website included Dr. Rochelle Walensky, director of the Centers for Disease Control and Prevention (CDC) and Dr. Anthony Fauci, chief medical advisor to President Biden and director of the National Institute of Allergy and Infectious Diseases (NIAID).
“The available data make very clear that protection against SARS-CoV-2 infection begins to decrease over time following the initial doses of vaccination, and in association with the dominance of the Delta variant, we are starting to see evidence of reduced protection against mild and moderate disease,” the statement read. “Based on our latest assessment, the current protection against severe disease, hospitalization and death could diminish in the months ahead, especially among those who are at higher risk or were vaccinated during the earlier phases of the vaccination rollout. For that reason, we conclude that a booster shot will be needed to maximize vaccine-induced protection and prolong its durability.
The current approval only includes the Pfizer and Moderna vaccines. Officials said they expect more data on Johnson & Johnson in the next few weeks. With that data in hand, they promise to keep the public informed with a timely plan for J&J booster shots as well.
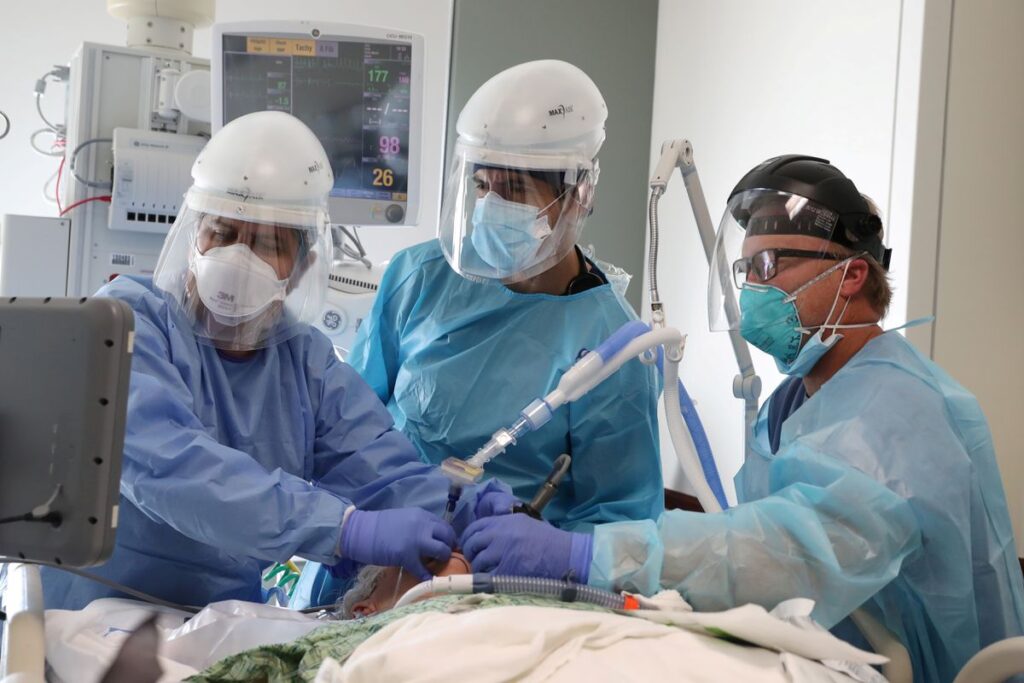
Doctors intubate a COVID-19 patient in the COVID-19 ICU at Providence Mission Hospital in Mission Viejo, California, Jan. 8. Photo: Lucy Nicholson/Reuters
The current protection against severe disease, hospitalization and death could diminish in the months ahead, especially among those who are at higher risk or were vaccinated during the earlier phases of the vaccination rollout – Statement from U.S. health officials
Wednesday’s statement said the government would also begin efforts to deliver booster shots directly to residents of long-term care facilities at that time, given the distribution of vaccines to this population early in the vaccine rollout and the continued increased risk that COVID-19 poses to them
On Wednesday, officials at Henry Ford Health System advised people who are immunocompromised not to wait to get a COVID-19 vaccine booster. Recommended boosters should be given at least 28 days after the second dose for those people. People should receive the same vaccine as their first two doses.
Immunocompromised told not to wait
The CDC recommends the booster for those who have moderately or severely compromised immune systems. This includes people in active cancer treatment, organ transplant or stem cell transplant recipients, and those with an advanced or untreated HIV infection. These individuals make up about 3 percent of the adult population and are especially vulnerable to COVID-19 because they are more at risk of serious, prolonged illness, the CDC says.
“Studies showed that people with this degree of immunosuppression, where their white blood cells don’t fight off infections quite as well, they always don’t get great immunity with two doses of the vaccine,” said Dr. Dennis Cunningham, system medical director of infection control and prevention at Henry Ford Health Systems. “When you give a third dose of vaccine these antibodies start appearing, these protective proteins, and it made a huge difference in immunocompromised people.”
eligible immunocompromised people also include those undergoing active treatment with high-dose corticosteroids or other drugs, and people with moderate or severe primary immunodeficiency such as DiGeorge syndrome and Wiskott-Aldrich syndrome.
COVID-19 related hospitalizations across Henry Ford’s hospitals have increased four-fold in recent weeks.
Dr. Cunningham said the booster is “safe, effective and well tolerated” and that Henry Ford is finalizing plans for administering boosters to its immunocompromised patient population, adding that local pharmacies and some municipalities are also offering it. The hospital system said it would be formalizing a plan in the coming weeks to administer booster shots to the general population.
Hospital officials said hospitalizations continue to rise. Dr. Adnan Munkarah, executive vice president and chief clinical officer, said COVID-19 related hospitalizations across Henry Ford’s hospitals have increased four-fold in recent weeks. In addition to 88 patients hospitalized with COVID-19, another 15 patients are admitted awaiting COVID-19 test results. The positivity rate is 9.4 percent, a significant increase from the 3.6 percent seen on July 21.
Munkarah said the Delta variant is driving the surge in hospitalizations among mostly unvaccinated people. Henry Ford has seen a small number of breakthrough infections involving immunocompromised patients or the frail elderly.
But Munkarah also said an upward trend in vaccinations has offered a “glimmer of hope.” Henry Ford has administered on average about 1,500 doses of vaccine per week for the past four weeks. With the FDA expected to give final approval to the Pfizer vaccine in the next few weeks, Munkarah said the upward trend of vaccinations should continue. He also advised those who are reluctant or hesitant to seek out trusted sources for reliable information about the vaccines.
Some 65 percent of people over 16 years of age in Michigan have had at least one shot of a COVID-19 vaccine, as of Tuesday. Close to 55 percent of Michigan’s population older than 12 years of age have been fully vaccinated.





Leave a Reply