LANSING — With the eligibility criteria for antivirals expanding to include more at-risk Michiganders, state health officials are urging those who test positive for COVID-19 to speak to their health care provider about treatment with oral medication.
The Michigan Department of Health and Human Services (MDHHS) says that the supply of oral medications to treat COVID-19 is expanding across the state. Health experts say it is a good idea for those with COVID to ask a doctor if this is the correct treatment for them.
Paxlovid and molnupiravir, which recently received emergency use authorization by the FDA, are designed for the treatment of mild to moderate COVID-19. Both medications may only be prescribed for patients by physicians, advanced practice registered nurses and physician assistants.
The MDHHS says that when administered to non-hospitalized patients with conditions that put them at high risk of severe illness within five days of symptom onset, these antivirals may reduce symptoms and the risk of hospitalizations and emergency department visits associated with the virus.
Although Michigan has received additional courses of the medications, “Priority Eligibility Criteria” for therapeutics, including antiviral medication and monoclonal antibody (mAb) therapy like sotrovimab, will remain in effect until the limited supply is able to meet demand and will be periodically reviewed as appropriate.
The MDHHS continues to strongly recommend getting vaccinated and boosted for the best protection against the virus.
Treatment with mAb continues to be an important therapy for mild to moderate COVID-19 infection and is preferred over treatment with molnupiravir whenever it can be readily accessed.
Based on current evidence, mAb therapy is also a comparable alternative to paxlovid for patients who do not have access to the oral medication, have contraindications to the medication or are beyond five days (but within 10 days) of symptom onset.
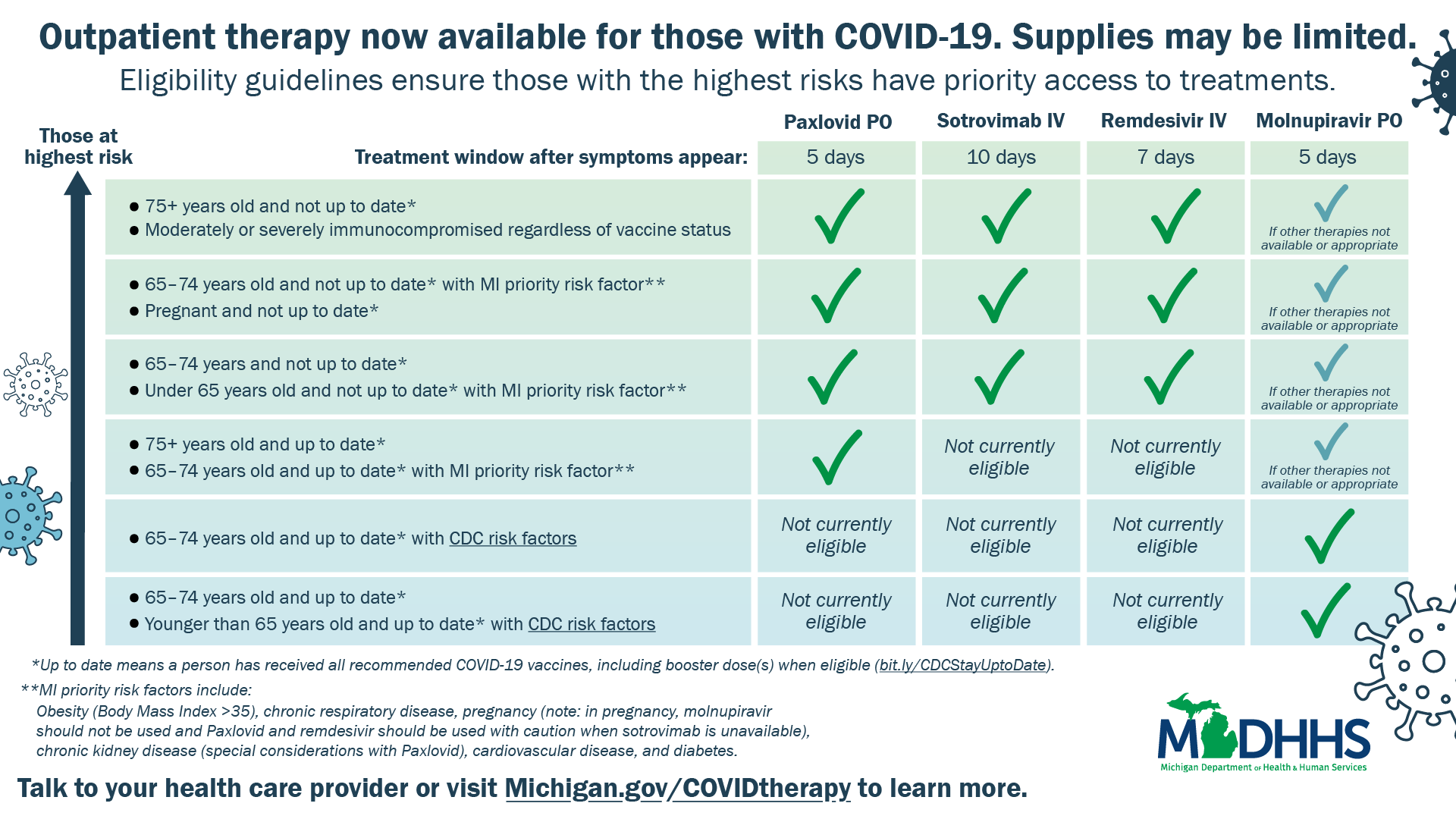
Image courtesy: The MDHHS
Paxlovid is indicated for the treatment of mild-to-moderate COVID-19 in patients 12 years of age and older who are at high risk for progression to severe COVID-19, including hospitalization or death, and who meet the current Priority Eligibility Criteria.
Paxlovid currently has limited availability through the following sites:
- Selected federally qualified health centers and tribal health centers
- Selected Meijer pharmacies throughout Michigan
- Selected retail pharmacies in areas not served by Meijer
Molnupiravir is indicated for the treatment of mild-to-moderate COVID-19 in adults ages 18 and older who are at high risk for progression to severe COVID-19, including hospitalization or death, and only when alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate and who meet the current Priority Eligibility Criteria.
Molnupiravir currently has limited availability through the following sites:
- All Meijer pharmacies
- Selected retail pharmacies in areas not served by Meijer
These medications are available at no cost to patients. Additional information on oral antiviral medications and monoclonal antibody therapy, including Priority Eligibility Criteria based on MDHHS scare resource allocation principles, is available at Michigan.gov/COVIDTherapy.





Leave a Reply